Human Reliability are specialists when it comes to human factors in medical device design including human factors and usability engineering, providing comprehensive solutions that optimize the design of medical devices, enhance user interactions, and minimize the risk of errors.
Human Reliability are specialists when it comes to human factors in medical device design including human factors and usability engineering, providing comprehensive solutions that optimize the design of medical devices, enhance user interactions, and minimize the risk of errors.
In the field of medical device design, human-centred approaches are essential to ensure the safety, effectiveness, and usability of healthcare technologies.
At Human Reliability Associates, our expertise and innovative methodology help medical device manufacturers meet industry regulations and deliver products that meet the highest standards of usability and safety.

In the field of medical device design, human-centred approaches are essential to ensure the safety, effectiveness, and usability of healthcare technologies.
At Human Reliability Associates, our expertise and innovative methodology help medical device manufacturers meet industry regulations and deliver products that meet the highest standards of usability and safety.
The medical device industry is subject to strict regulations and standards. One such widely recognized standard is 62366, which focuses on usability engineering.
Compliance with this standard is crucial for ensuring that medical devices are designed with the end-user’s needs in mind. Our Human Factors and
Usability Engineering Services are aligned with these regulations, offering a systematic approach to identify, mitigate, and manage usability issues throughout the device development lifecycle.
By applying user-centred design principles, conducting task analyses, and employing human factors testing methodologies, we help you meet regulatory requirements and create devices that are safe, effective, and intuitive for end-users.

The medical device industry is subject to strict regulations and standards. One such widely recognized standard is 62366, which focuses on usability engineering.
Compliance with this standard is crucial for ensuring that medical devices are designed with the end-user’s needs in mind. Our Human Factors and
Usability Engineering Services are aligned with these regulations, offering a systematic approach to identify, mitigate, and manage usability issues throughout the device development lifecycle.
By applying user-centred design principles, conducting task analyses, and employing human factors testing methodologies, we help you meet regulatory requirements and create devices that are safe, effective, and intuitive for end-users.
Risk management is another critical aspect of medical device design. Compliance with standard 14971 for reducing risks in medical devices is essential for ensuring patient safety. Our Human Factors and Usability Engineering expertise allows us to contribute to the risk management process by identifying and addressing potential risks associated with human interactions and device usability.
Through a combination of thorough task analysis, hazard identification, and risk assessment, we help you minimize the occurrence of errors and mitigate risks throughout the lifecycle of your medical device.
Risk management is another critical aspect of medical device design. Compliance with standard 14971 for reducing risks in medical devices is essential for ensuring patient safety. Our Human Factors and Usability Engineering expertise allows us to contribute to the risk management process by identifying and addressing potential risks associated with human interactions and device usability.
Through a combination of thorough task analysis, hazard identification, and risk assessment, we help you minimize the occurrence of errors and mitigate risks throughout the lifecycle of your medical device.

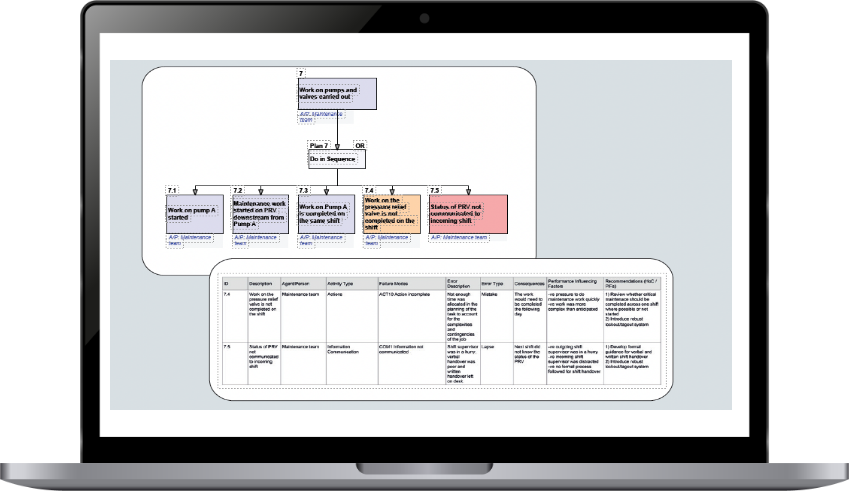
At Human Reliability Associates, we have developed a powerful methodology that helps medical device designers streamline their design processes and enhance usability.
Our approach involves a task analysis that maps out user interactions and identifies potential interaction problems and areas where errors could occur. By addressing these issues at the design stage, we can proactively improve usability and prevent errors.
Our innovative SHERPA software has proven to be a game-changer, making task analysis up to seven times faster for medical device designers.
Unlike traditional uFMEA methods, our SHERPA methodology offers a more systematic and powerful way to analyze tasks, identify potential issues, and implement effective design improvements.

At Human Reliability Associates, we have developed a powerful methodology that helps medical device designers streamline their design processes and enhance usability.
Our approach involves a task analysis that maps out user interactions and identifies potential interaction problems and areas where errors could occur. By addressing these issues at the design stage, we can proactively improve usability and prevent errors.
Our innovative SHERPA software has proven to be a game-changer, making task analysis up to seven times faster for medical device designers.
Unlike traditional uFMEA methods, our SHERPA methodology offers a more systematic and powerful way to analyze tasks, identify potential issues, and implement effective design improvements.
With our deep expertise in Human Factors and Usability Engineering for medical device design, Human Reliability Associates is your trusted partner in optimizing usability, enhancing safety, and ensuring regulatory compliance.
Our comprehensive solutions, including task analysis, risk management, and the innovative SHERPA software, enable you to create medical devices that deliver exceptional user experiences and improve patient outcomes.


With our deep expertise in Human Factors and Usability Engineering for medical device design, Human Reliability Associates is your trusted partner in optimizing usability, enhancing safety, and ensuring regulatory compliance.
Our comprehensive solutions, including task analysis, risk management, and the innovative SHERPA software, enable you to create medical devices that deliver exceptional user experiences and improve patient outcomes.
This video demonstrates the use of the SHERPA methodology and software, which until recently was called the Human Factors Risk Manager. This shows how we can develop a detailed task analysis, how we can predict potential errors in the task, and identify factors influencing the likelihood of those errors.
This video demonstrates the use of the SHERPA methodology and software, which until recently was called the Human Factors Risk Manager. This shows how we can develop a detailed task analysis, how we can predict potential errors in the task, and identify factors influencing the likelihood of those errors.
Contact us today to discuss how our services can improve human factors in aviation operations and drive continuous improvement in human performance.
Get the latest newsletter by signing-up today





This free 30 minute mini-course will introduce you to Human Factors, and how critical task reviews are used to improve the quality and safety of tasks and processes across different industries.
It’s free, informative and you’ll even get a certificate of completion.

This short and engaging handbook provides a great overview of Human Factors Systems Critical Task Analysis (SCTA) and how it helps people across sectors reduce error and improve human performance.
SCTA can help keep people safe and delivers value.


Popular Pages
© Human Reliability 2025.
1 School House, Higher Lane,
Dalton, Lancashire,
WN8 7RP, UK.
T: +44 (0) 1257 463 121
E: [email protected]