The Parable of the Ant: Context Shapes Behaviour

“Context shapes behaviour” – This fundamental principle in human performance is brilliantly illustrated through the Parable of the Ant. This blog traces this powerful metaphor from Herbert Simon’s original insight to modern human factors applications.
Using SEIPS to Understand and Improve Patient Safety
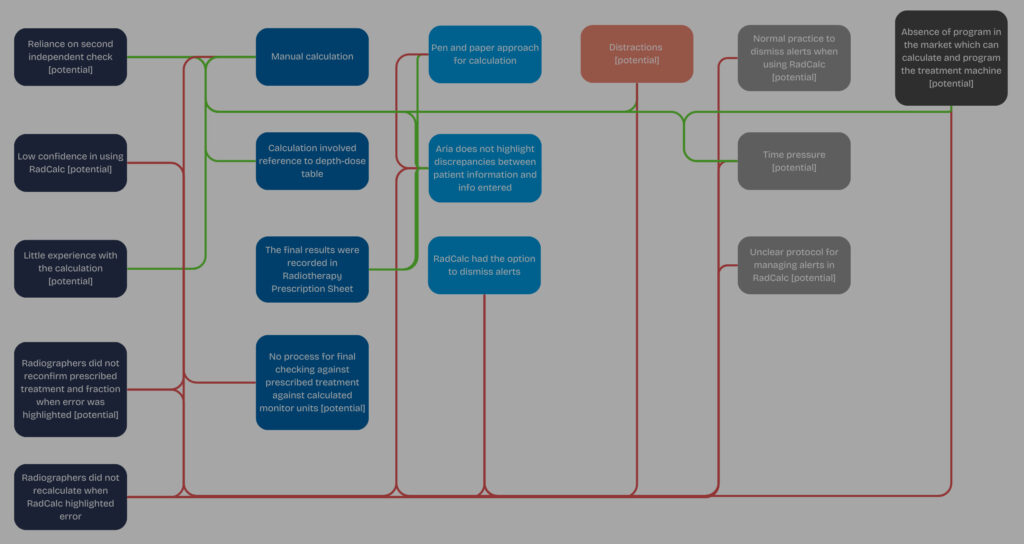
Lydea’s latest blog explores how the Systems Engineering Initiative for Patient Safety (SEIPS) framework help move human error beyond individual actions to systemic factors that contribute to such incidents.
The blog identifies key vulnerabilities and explores potential opportunities for improvement to enhance patient safety.
Revisiting the Swimlane Method: A Healthcare Case Study to Improve Patient Safety

Lydea’s latest blog explores how Swimlane could be applied in the healthcare industry. By mapping out the sequence of events, the tool helped uncover opportunities to improve processes and enhance safety.
Tackling Procedural Non-Compliance in the Workplace: Insights and Solutions
Standard Operating Procedures (SOP) are essential, but why do operators deviate from them? Our Managing Director, David Embrey, explores how non-compliance may not always be about negligence, but rather systemic challenges and opportunities for process improvement.
Human Factors Regulations in Singapore: A comparison with the UK

In 2017, Singapore introduced the Safety Case Regime under the Workplace Safety and Health (Major Hazards Installations) Regulations, marking a pivotal step toward strengthening safety at facilities handling hazardous substances. This approach aligns closely with the UK’s HF Delivery Guide under the Control of Major Accident Hazards (COMAH) Regulations.
Human Performance Guidance for Pharmaceutical Manufacturing: A Regulatory Innovation?

In the pharmaceutical sector, human error isn’t just a “mistake”—it can affect patient safety, production and quality.
Human Factors for Biological Containment Labs

Biological containment labs handle some of the most dangerous pathogens, yet human error remains a critical risk factor in their operations.
Failure Modes and Mechanisms in Safety Critical Task Analysis (SCTA)

In the realm of safety critical tasks industries, identifying both failure modes and mechanisms is essential for effective risk management and performance improvement. We dive into this crucial topic, exploring how failure modes and failure mechanisms impact human performance.
Understanding the HSE’s Human Factors Delivery Guide for COMAH Sites: A Quick Overview for Beginners
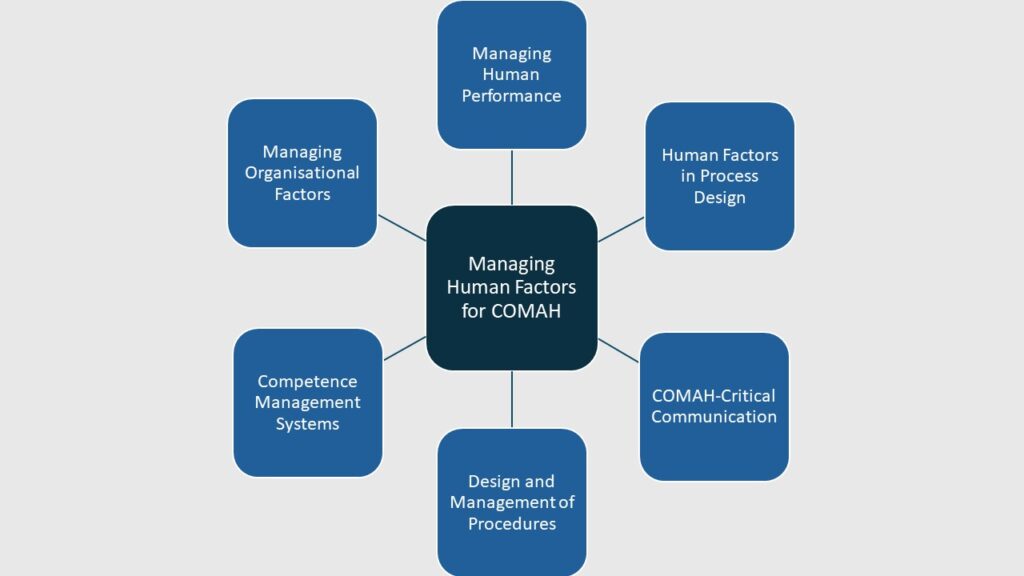
Want to learn more about the 6 topic areas in the HSE’s HF COMAH Delivery Guide? Here, we give a quick overview of these areas and how they can you help improve Human Factors management.
CAPA Culture and systems: Organisational learning in pharmaceutical manufacture

At HRA, we have found that reporting culture isn’t quite as healthy as we might hope, both in healthcare and other industries.