A clarion call for pharmaceutical manufacturers is the reduction of human error for the sake of quality, production and patient safety. Regulatory guidance on human performance also plays a larger and more mature role in other sectors – what can be learnt to improve the state of the art for pharmaceutical manufacturing?
Pharmaceutical and high-hazard industries, like chemical processing or oil and gas, share common risks related to human performance in critical safety and quality roles. Drawing parallels between these sectors could offer the pharmaceutical industry and regulators a fresh approach and insight to reducing the likelihood of human error, which is often a key factor in deviations from the documented process (EudraLex Vol 4 Chap 1[i]).
The idea we explore in this article is for pharmaceutical regulators to be inspired by the structure and principles from the Control of Major Accident Hazards (COMAH) Human Factors Delivery Guide.
Why Human Performance Matters in Pharmaceuticals
In the pharmaceutical sector, the consequences of human error range from product recalls to regulatory violations, and potentially to adverse health outcomes for patients. However, many strategies to mitigate these risks focus primarily on technology, automation, and process control, often overlooking the critical role of human performance and interaction within the system. The strategies often see people as the problem to solve rather than understanding they are the solution to harness. (Dekker, 2014)[ii]
Similar to the high-hazard sectors regulated under COMAH, the pharmaceutical industry relies heavily on people performing critical tasks and managing critical risks—whether it’s in sterile manufacturing environments, clinical trials, or drug quality assurance.
Traditionally Pharmaceutical Manufacturing rely on a paper documented quality management system to demonstrate how they control processes where people are expected to remember every line, which is not always possible.
The documented processes are however, only part of the system. So, it is for some a significant shift in mindset to learn how to describe human interactions with the system (not just human actions for individual processes). Using Human Factors helps an organisation learn and better recognise how humans really achieve successful execution of the processes and engage the system as a whole to reliably deliver the required outcomes. How we think the process and system works (work-as-imagined) is often different to how it performs (work-as-done).
Brief background to Human Factors for COMAH sites in the UK
Human Factors play a critical role in safety and operational performance at COMAH (Control of Major Accident Hazards) sites in the UK, where the risks of industrial accidents can have serious implications. This focus on Human Factors was strengthened after major incidents like the Flixborough explosion in 1974, the Piper Alpha disaster in 1988, and the Buncefield oil storage terminal explosion in 2005, which highlighted the need for a systemic approach to prevent human errors in high-hazard industries.
The Competent Authority Inspection & Review Group (CAIRG) led a review to establish robust guidance and frameworks for human factors, refining the broad field into six key topics that are essential for safety in these environments:
- Managing Human Performance
- Human Factors in Process Design
- COMAH-Critical Communications
- Design and Management of Procedures
- Competence Management Systems
- Managing Organisational Factors
The UK’s COMAH human factors approach is one of the most mature in the world, with other countries following its lead. It provides detailed success criteria for regulators to assess site safety, alongside practical guidance for operators to implement these best practices effectively.
The Control of Major Accident Hazards (COMAH) Human Factors Delivery Guide is freely available from the regulator’s website.
How Pharmaceutical Regulators might consider drafting their Own Human Performance Guidance for Pharmaceutical Manufacturers
Drawing from COMAH’s structure, pharmaceutical regulators could consider their own version of a human performance delivery guide. We reflect on themes from COMAH and Human Factors which should be considered when developing new guidance:
1. A Systems-Based Approach to Human Error
The COMAH delivery guide emphasizes that human error is often symptomatic of broader systemic failures rather than isolated mistakes. Pharmaceutical regulators could consider increasing the expectation of this holistic approach to understanding errors (EudraLex Vol 4.1.4[iii]).
Exploring how factors like how the work is organised, workload, procedural design and accessibility, effectiveness of controls, communication and feedback, competence (formalised and risk-informed knowledge, skills and experience rather than more informal apprentice type training wrapped up in organisational culture) affect human performance.
Focusing on what influences performance, the underlying causes of errors, regulators can guide industry to promote deeper, more sustainable improvements in compliance and safety. This systems-based approach can also be used for proactive and predictive risk assessments.
2. Understanding the critical risks, going beyond Risk Assessment
Under COMAH, human performance is integrated into risk assessments for critical tasks, highlighting high risk areas e.g. handovers, communication, focusing not just on technical risks but improving the ability to anticipate and mitigate potential human errors and their contributary causes.
Pharmaceutical regulators could encourage companies to apply the same proactive mindset to Quality Management Systems (ICH Q-10)—considering how the design of processes, roles, and environments could set the stage for challenges to human performance and make human error more likely (error traps). Visibility of these potential error traps enables the organisation to apply mitigations before problems occur.
ICH Q9[iv] includes a list of Human Factors tools and recommended human performance approaches in the Appendices. Recognizing that individuals manage risks daily—often through informal, tacit ‘tribal knowledge’—could help mitigate the limitations this practice imposes on organizational learning and improvement.
Without anticipating and learning about the potential for human failures in foresight will leave us merely to learn about them in hindsight. Under COMAH, detailed and systematic Human Reliability Assessment (HRA) is required for critical tasks.
3. Building Layers of Defence – the human contribution
In high-hazard industries, no single control is considered foolproof, and multiple, independent layers of defence are put in place to mitigate the likelihood of human error.
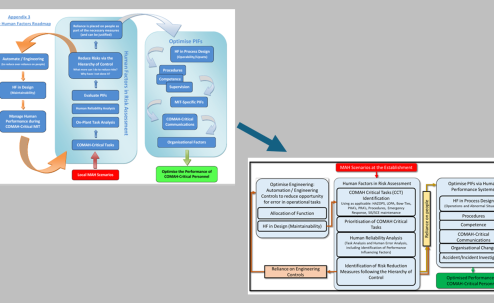
Pharmaceutical regulators could provide guidance and expectations about similar controls in manufacturing processes. Under COMAH, the Hierarchy of Control is applied to critical tasks, to help control risks. This could involve simplifying tasks, adding engineering controls, alarms and alerts. From this perspective procedural controls and training are towards the bottom of the Hierarchy of Control – so they are seen as some of the least effective forms of control.
Part of the opportunity to improve the effectiveness of defences is by including greater emphasis on understanding how work is actually done, what it takes for people to deliver work not just what the SOP states.
4. Human Factors Awareness
Integrating a stronger emphasis on Human Factors training into compliance expectations for pharmaceutical regulators can enhance operators’ and managers’ understanding of risk management in day-to-day tasks. This approach can also improve their handling of deviations, nonconformities, and other operational challenges. By fostering risk-based decision-making and clear communication, organizations can better learn from these experiences and continuously improve
More efficiently and cost effectively the same training, approaches and tools can be applied to continual improvement (key objective of ICH Q-10[v]– Quality Management Systems)
5. Learning from Incident Investigations
Human error is a consequence rather than a cause of incidents. Declaring human error as a cause is not acceptable from a Human Factors perspective and reflects inspection feedback and findings
Learning from incidents should aim for effective and sustainable solutions to prevent future system mistakes. For example, if CAPAs focus on updating the SOP, which are rarely used in hand then this might not be an effective intervention.
More effective learning from investigations happens when people who do the job are involved. Focus should also be on understanding how the system set the stage (antecedents) for human error and if applicable how the system failed to catch or mitigate the error. Pharmaceutical regulators could adopt a similar approach, requiring companies to look into what factors influenced human performance when compliance issues, product recalls, or drug safety incidents occurred to understand both the human and systemic contributions to the failure. This is sometimes referred to as ‘learning teams’ in the industry based on Peter Senge’s ‘Learning Organisation’[vi].
Conclusion
While pharmaceutical and high-hazard industries may seem worlds apart, they share a common challenge: managing human performance in high-risk environments. By drawing lessons from the COMAH delivery guide, pharmaceutical regulators could enhance how they promote setting people up for success and the resulting reduction in likelihood of human error, improving safety and compliance across the industry. Ultimately, this would lead to making work easier and helping to provide better protection for patients and a stronger, more resilient pharmaceutical sector.
Next steps… We would welcome a dialogue between industry, regulators and relevant professional bodies to design and implement new guidance. For example, BioPhorum has already done important work for managing human performance for the pharma and biopharma manufacturing, and the CIEHF (Chartered Institute of Ergonomics and Human Factors) has an active Pharma Manufacturing group ready to engage.
If you would like to learn more about the Human Factors topic within the COMAH HF Delivery Guide, we’ve published a blog explaining each topic here.
Julie chairs the CIEHF’s Pharma Manufacturing group. If you would like to join this group, please get in touch with her at [email protected]
[i] EudraLex Volume 4, Chapter 1, 1.8 , point (vii) “Any significant deviations are fully recorded, investigated with the objective of determining the root cause and appropriate corrective and preventive action implemented” GMP Guide Chapter 1 Q10 implementation final. Deviations are sometimes categorised as minor, major and critical
[ii] Dekker, S. (2014). Safety Differently: Human Factors for a New Era. 2nd ed. CRC Press.
[iii] EudraLex Volume 4, Chapter 1, 1.4, point (xiv) “Where human error is suspected or identified as the cause, this should be justified having taken care to ensure that process, procedural or system based errors or problems have not been overlooked, if present. Appropriate corrective actions and/or preventative actions (CAPAs) should be identified and taken in response to investigations. The effectiveness of such actions should be monitored and assessed, in line with Quality Risk Management principles.”
[iv] European Medicines Agency, “ICH Guideline Q9 on Quality Risk Management ,” 2015. Available: https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use-ich-guideline-q9-quality-risk-management-step-5-first-version_en.pdf
[v] European Medicines Agency, “ICH Guideline: Q10 on Pharmaceutical Quality System,” 2015. Available: https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-guideline-q10-pharmaceutical-quality-system-step-5_en.pdf
[vi] P. Senge, The Fifth discipline: the Art and Practice of the Learning Organization. London: Random House Business, 2006.